ProofPilot is the industry's only Digital Protocol Automation platform for Recruitment and Conduct. ProofPilot eliminates burden, guesswork and protocol deviations to create high-performance experiences for your sites and patients.

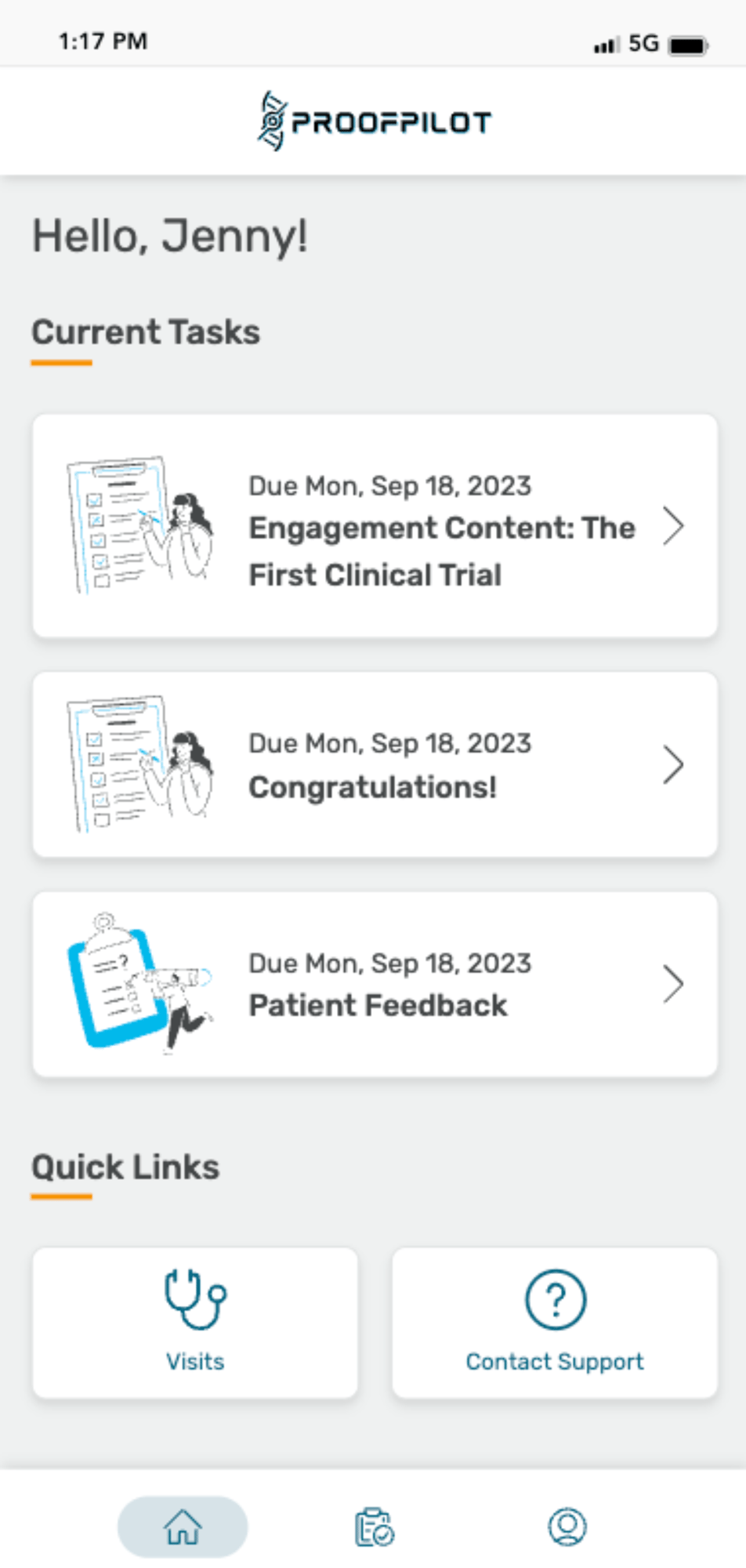
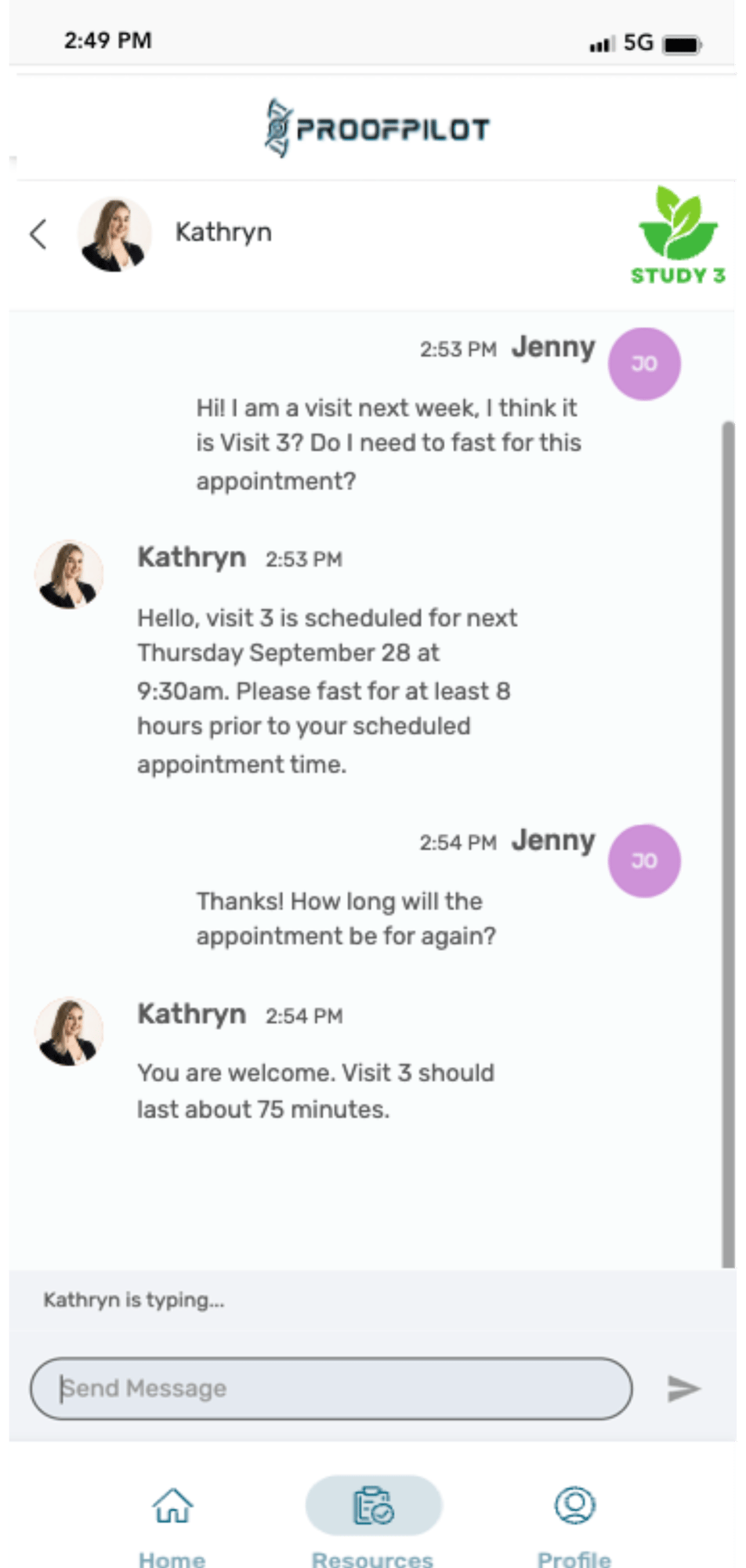
The Protocol Automation Platform
Don’t let manual tasks and paper protocols cause complexity, burden, inefficiencies and bad data in your clinical trials. Let ProofPilot’s protocol automation platform seamlessly orchestrate all recruitment and study tasks for sites and patients on any device, anywhere.
Maximize Quality
Simplify the Complex
Save Time & Effort

Boost ROI
.png)
"We used PROOFPILOT to enroll 300 patients for DCT digital biomarker study – it delivered enormous cost savings, quality data, and great compliance.”
"PROOFPILOT ensured consistency, which was critical because of the subjective nature of the endpoints."
"PROOFPILOT was able to measure compliance at the question level of eDiaries and ePROs. Amazing!"
"Through integrated workflows to ensure timely training, logistics and patient payments, we saw nearly 90% compliance from our participants"


